
9.4.4 Plotting the climate space of the Zebra Finch.9.4.3 Plotting the climate space of the Desert Iguana.9.4.2 Plotting climate space boundaries for a cylinder with varing solar absorptivity.9.4.1 Defining a function for computing the bounding air temperature/radiation combinations.
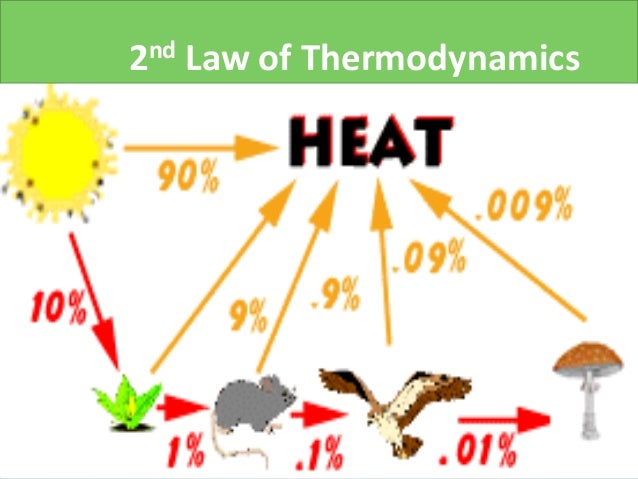
9.4 PHYSIOLOGICAL CONTRAINTS OF THE ORGANISM.9.3 THE THERMAL ENVIRONMENT: BASIS FOR THE CLIMATE SPACE.6.7.1 General Texts and Papers on Energy Budgets.6.3.3 The First Law Generalized to Include Mass Flow.6.3 APPLICATIONS OF THE FIRST LAW OF THERMODYNAMICS.4.5.4 Gravitational and Electrostatic Potential Energy.4.4.2 Other Force “Laws”–Friction, Intermolecular Forces, Hooke’s Law.4 Foundations of Physical Theory I: Force and Energy.3.4.2 INTENSIVE AND EXTENSIVE PROPERTIES.3.4.1 THE DIMENSIONAL CONSTRAINTS ON DEFINITIONAL AND EMPIRICAL EQUATIONS.3.3.6 AUXILIARY PREFIXES OF THE METRIC SYSTEM TO INDICATE DECIMAL MULTIPLES AND SUBMULTIPLES.3.3.3 SUPPLEMENTARY MECHANICAL UNITS (cgs and English systems).2.4.2 Critical Points in Three Dimensions.1.12 SOLUTION TO THE ADDITIONAL PROBLEMS.1.6.1 Accumulation of Changes in the Function.Due to entropy, which is the measure of disorder in a closed system, all of the available energy will not be useful to the organism.
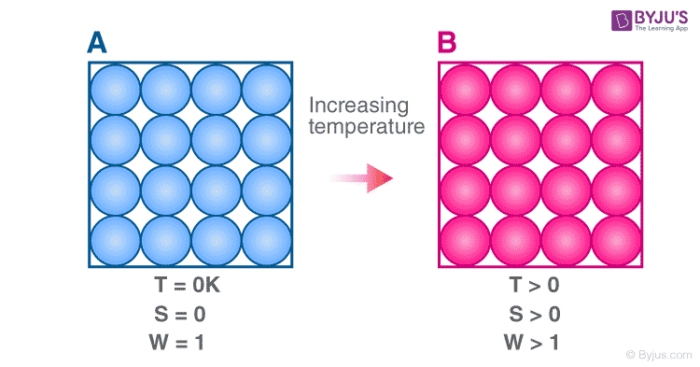
The Second Law of Thermodynamics states that when energy is transferred, there will be less energy available at the end of the transfer process than at the beginning. How does the second law of thermodynamics apply to biological systems? The second law states that the acceleration of an object is dependent upon two variables – the net force acting upon the object and the mass of the object. A cold object in contact with a hot one never gets colder, transferring heat to the hot object and making it hotter. What is the best example of the second law of thermodynamics?įor example, heat involves the transfer of energy from higher to lower temperature. The Second Law of Thermodynamics is universal and valid without exceptions: in closed and open systems, in equilibrium and non-equilibrium, in inanimate and animate systems - that is, in all space and time scales useful energy (non-equilibrium work-potential) is dissipated in heat and entropy is generated. Second law of thermodynamics is very important because it talks about entropy and as we have discussed, ‘entropy dictates whether or not a process or a reaction is going to be spontaneous’.ĭoes the second law of thermodynamics apply to open systems?
What is the second law of thermodynamics and why is it important? Some of the energy “leaks” away through friction and heat. The electricity is still pretty concentrated, but not all of the mechanical energy is converted to electricity. What is a real life example of the second law of thermodynamics?įor example, when a diesel engine turns a generator, the engine’s mechanical energy is converted into electricity. Entropy is zero in a reversible process it increases in an irreversible process. Another form of the second law of thermodynamics states that the total entropy of a system either increases or remains constant it never decreases. How does entropy relate to the second law of thermodynamics?Įntropy is the loss of energy available to do work. Ultimately, this is one of the key elements dictating an arrow of time in the Universe. The second law of thermodynamics states that entropy, which is often thought of as simple ‘disorder’, will always increase within a closed system. What is the second law of thermodynamics in simple terms?


 0 kommentar(er)
0 kommentar(er)
